Emerging technologies such as advanced analytics, robotics, and automation promise significant rewards for the pharmaceutical industry, including increased speed, compliance, cost savings, and productivity improvements.
Enhanced agility and reduced testing times can lower QC-lab lead times by 60 to 70 percent, paving the way for real-time releases. Digitization and automation also ensure better quality and compliance by minimizing manual errors and variability, and enabling faster, more effective problem resolution.
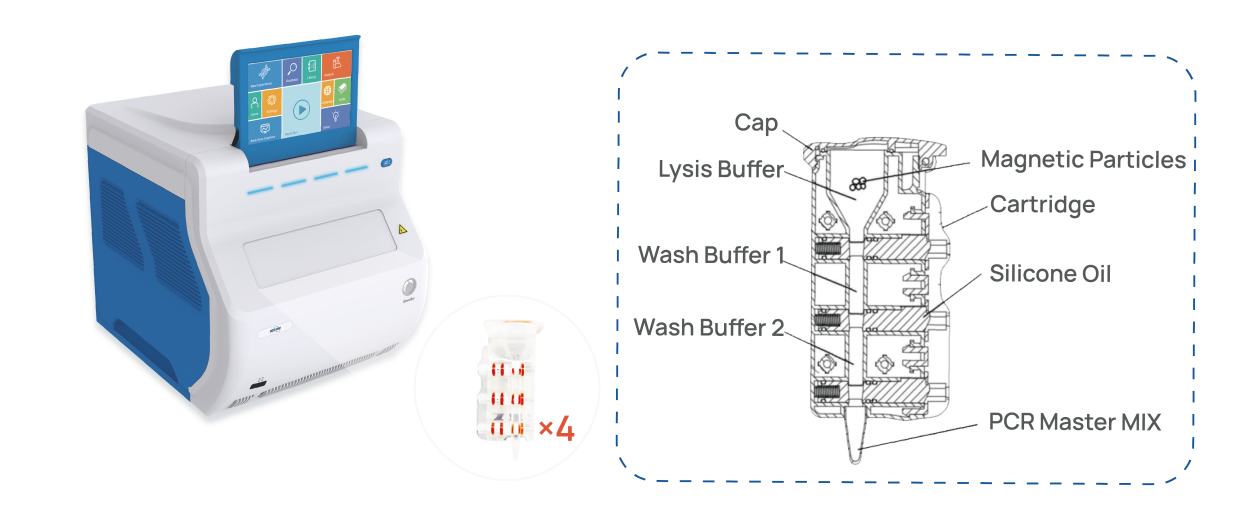
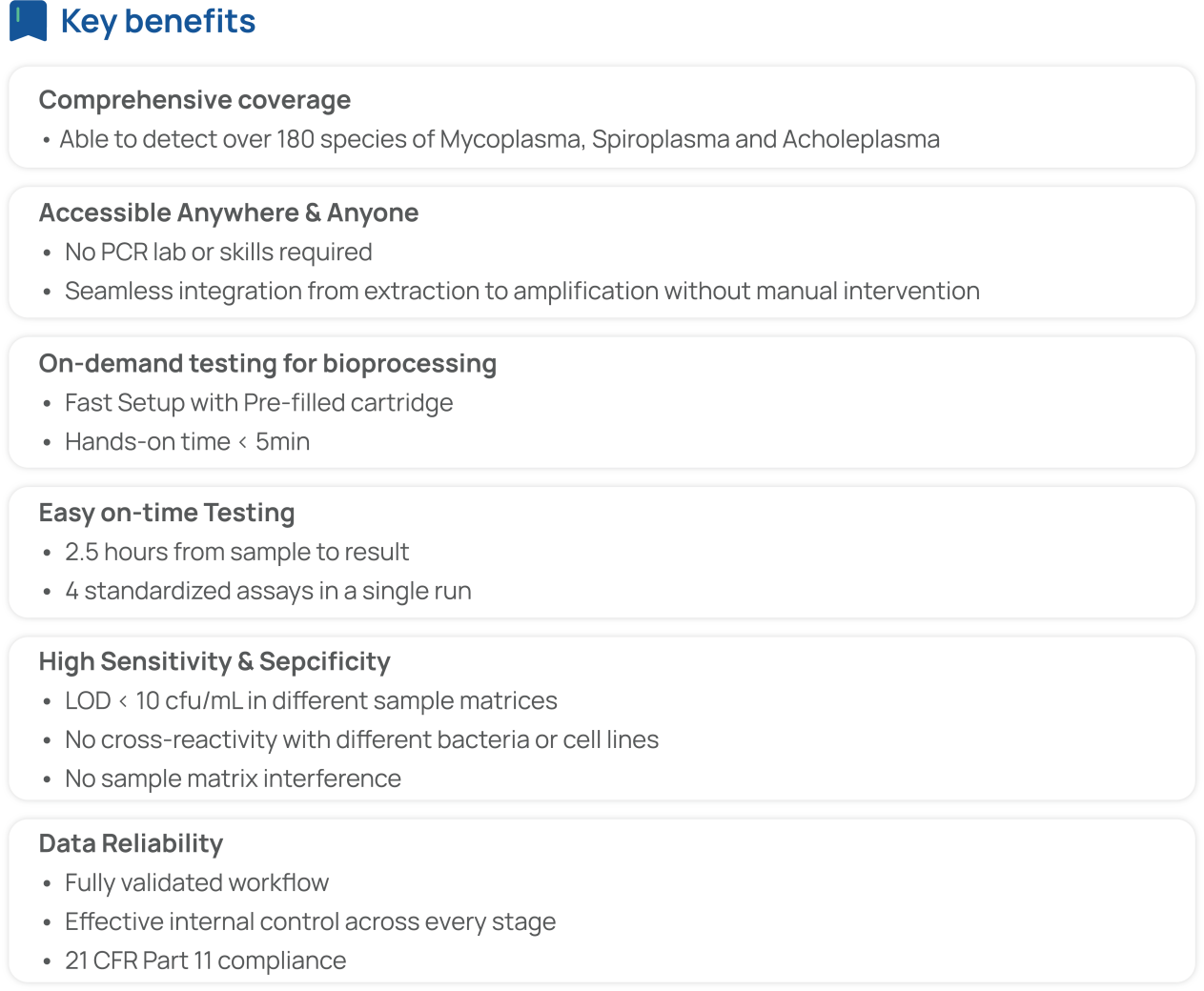
For example, the AdvSHENTEK Mycoplasma DetectInnova System revolutionizes rapid microbiological methods (RMM) with a streamlined, fast, fully automated, and portable laboratory system. It integrates nucleic acid extraction and real-time PCR assay, delivering results in just 2.5 hours from sample to result. Remarkably, it requires less than 5 minutes of hands-on time and no specific expertise in DNA extraction and PCR to operate.