Plasmid DNA (pDNA) are applied for gene therapy and vaccine development. The purification of DNA plasmids is difficult, because supercoiled pDNA (the product) is quite similar in size and structure to the contaminating RNA, genomic DNA and open-circular pDNA that are released upon cellular lysis. And the yield is a challenge for pDNA production, because pDNA constitutes less than 5% of the clarified bacterial lysate, with RNA constituting over 20% and proteins more than 50%.
Monitoring and controlling the levels of the residual RNA are essential to meet regulatory requirements and ensure patient safety. Compliant detection of host cell residual RNA is essential to maintain both product safety and workflow efficiency.
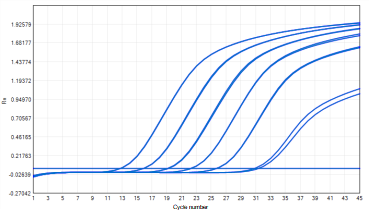
Real-time quantitative PCR technology, with its high sensitivity and specificity, has become the preferred method for detecting trace RNA. It accurately measures the quantity of specific RNA sequences in samples, thereby assessing the efficiency of the clearance process. NGS technology provides a more comprehensive approach, capable of detecting all RNA sequences in a sample, which is useful for comprehensive understanding of host cell expression profiles and discovery of non-target RNA.
The accuracy and efficiency of host cell residual RNA detection can provide strong support for drug development, as the demand for drug safety and efficacy is constantly increasing in the development of personalized and precision medicine.
E.coli RNA Controls - qPCR Amplification Plot