Host Cell Proteins (HCPs), originating from engineered cells in biopharmaceutical production, can potentially impact product quality, efficacy, and safety. Therefore, HCP monitoring constitutes a critical quality attribute (CQA) in the manufacturing process. Given the extensive diversity of HCPs, multiple detection and identification techniques are required to satisfy global regulatory standards.
1. HCP Analysis and Detection Technology Platform (ELISA、LC-MS & 2D)
a. SHENTEK® HCP ELISA kits customized to a specific technique to ensure precise and targeted detection.
b. HCP ELISA standards traceability system to ensure assay precision and reliability.
c. Accurately assess process-specific and high-risk HCPs and the variations in HCPs between batches, such as preclinical and clinical trial lots.
d. Identify high-risk HCPs using orthogonal techniques to supplement HCP ELISA limitations for the bioprocesses development.
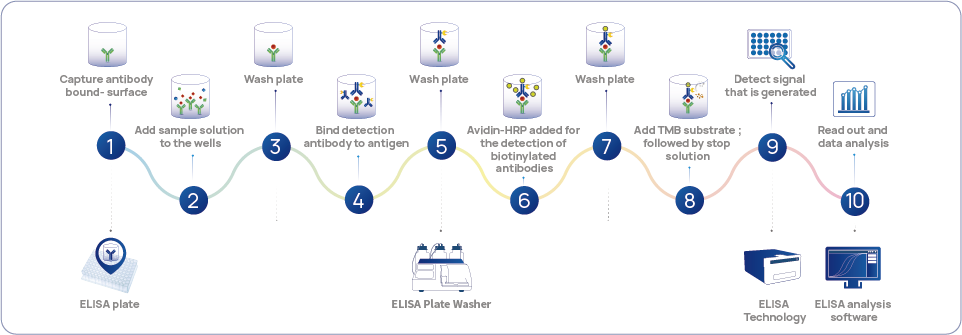
Figure 1 HCPs standard technology - ELISA detection workflow
2. HCP Antibody Coverage Analysis Platform (IMBS-2D and IMBS/LC-MS)
a. IMBS® (immunomagnetic bead separation) technology based on antigen-antibody-magnetic bead immune complexes for quick separation of specific antigens.
b. 2D and LC-MS offer a multi-angle, precise, and objective representation of the matching degree between antibodies and HCPs.
c. HCP antibody specificity is measured by coverage analysis on simulated or early-stage process samples.
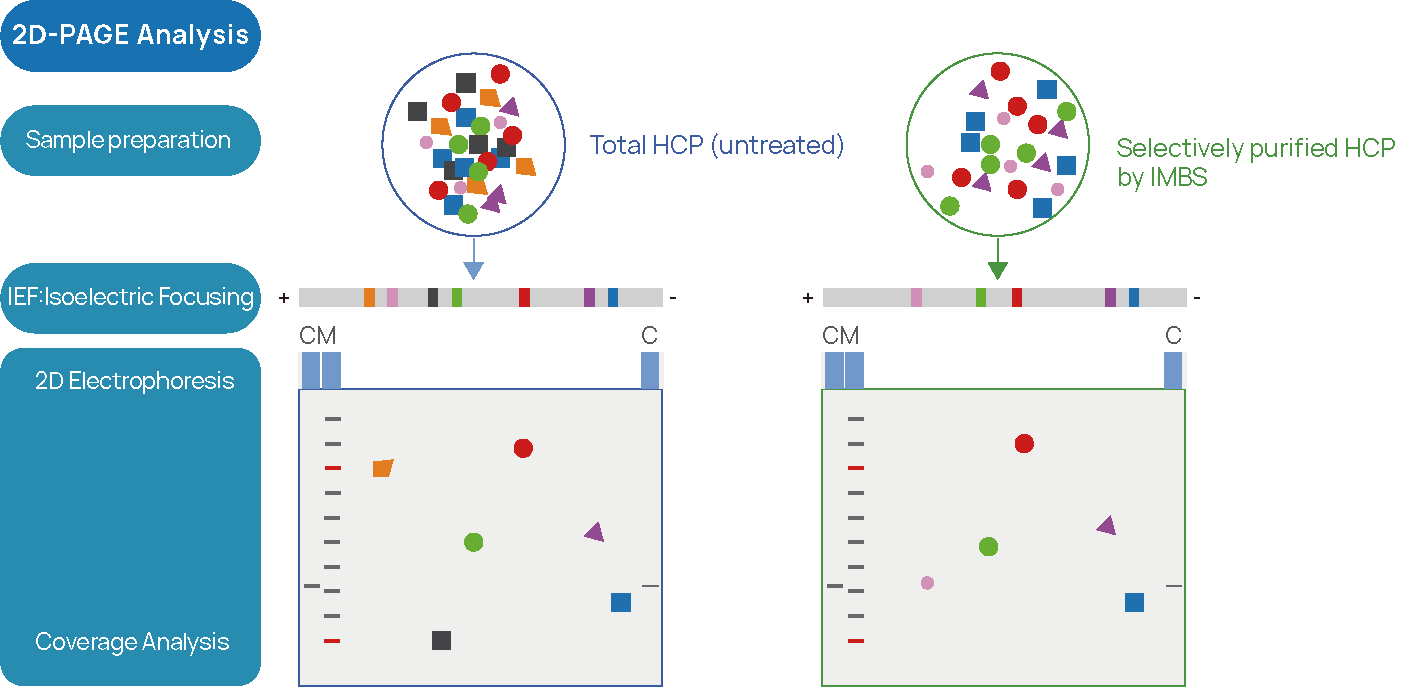
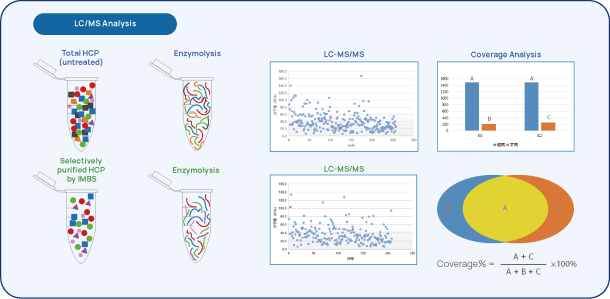
Figure 2 IMBS-2D and IMBS-LC/MS HCP-antibody Coverage Analysis
3. HCP Polyclonal Antibody Preparation Platform
a. Customize immune pathways and strategies for different antigens.
b. Monitor the preparation of high-quality antibodies with precise antibody characterization through the whole process.
c. A multi-mode standardized antibody preparation platform to ensure the generation of antibodies with high coverage rate and efficacy.
d. Ensure the reliability and comparability of the detection system through the traceability system of HCP reference products.
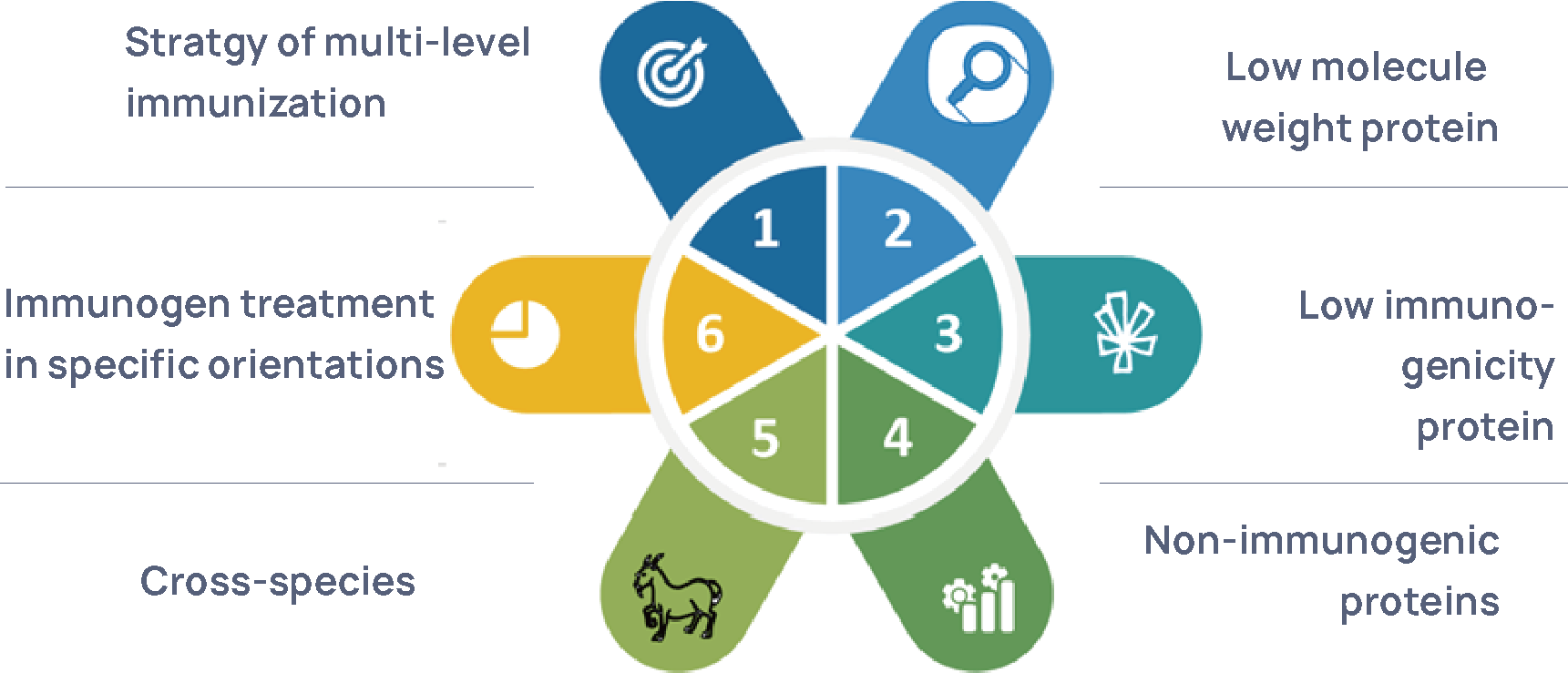
Figure 3 HCP Polyclonal Antibody Preparation Platform
4. Customized HCP ELISA Kit Platform
a. Comprehensive studies of residual HCP (rHCP) standards to achieve excellent coverage and specificity.
b. Standard practice of HCP reference traceability to ensure the reliability of immunoassays.
c. Effective and intensive antigen and antibody preparation strategies for robust immune response and high-quality polyclonal antibodies.
d. Compliance with ISO13485 quality system to guarantee the high-quality HCP ELISA kit development.
e. Compliance with CNAS/ISO10725 and GMP quality standards to ensure the integrity of the service and products.
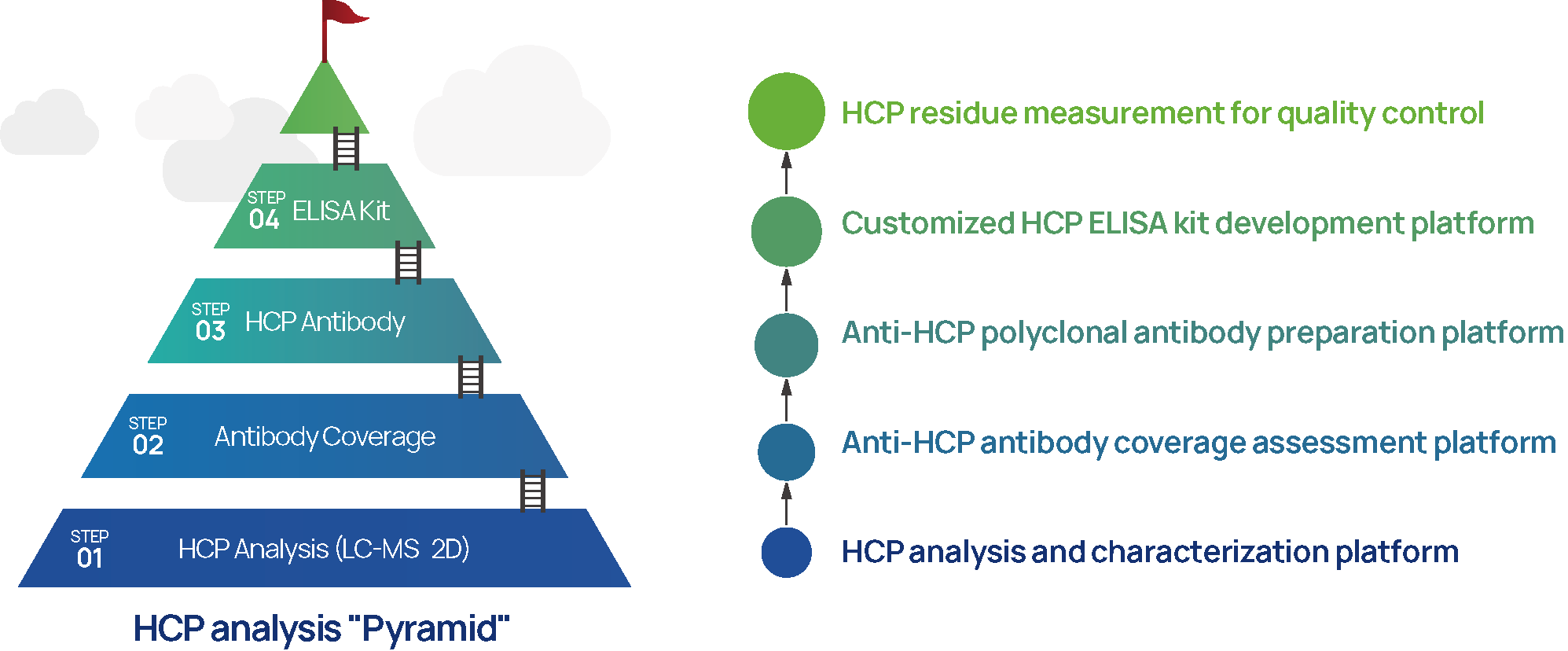
Figure 4: SHENTEK Residual Host Cell Protein Analysis Service Platforms