QC Solutions to Empower Your Research
SHENTEK is always committed to providing standardized and customized high-quality QC solutions and products. With over 10 years' experience, SHENTEK has developed overall product lines, covering host cell DNA, RNA, protein, CAR gene, bacteria, Mycoplasma, virus, cell line, process-related residue, etc. to meet the QC needs from starting and raw materials to in-process samples and final products.

We provide our customers with standardized and customized solutions, that adhere to international bioindustry standards to ensure compliance and satisfaction.

Efficient order processing, handling, packaging, warehousing, and delivery for seamless logistics.

Extensive production pipelines have been developed, validated and monitored on the entire production process from material to delivery and the products met specifications.

Facilities operations and maintenance encompasses the day-to-day activities and cleaning, maintain systems, contamincation control with approved procedures and instructions.
To deliver value to our customers, we deeply understand customer needs and shape our business.

We ensure appropriate training and guidance to staff, pursue the best way to ensure compliance.
Digitally-enabled labs use real-time analytic and process verification to optimize operations, reduce workload by 90%, improve compliance and accelerate laboratory efficiency. QC team can identify any deviations or contaminants that could compromise product safety through rigorous testing of raw materials, in-process samples, and finished products.
Automated labs use robots and advanced automation to streamline repetitive tasks. Pharmaceutical companies use automated equipment can expedite efficiency, reduce downtime with remote monitoring, shortening lab lead time by 75%, enabling rapid and intelligent drug production.
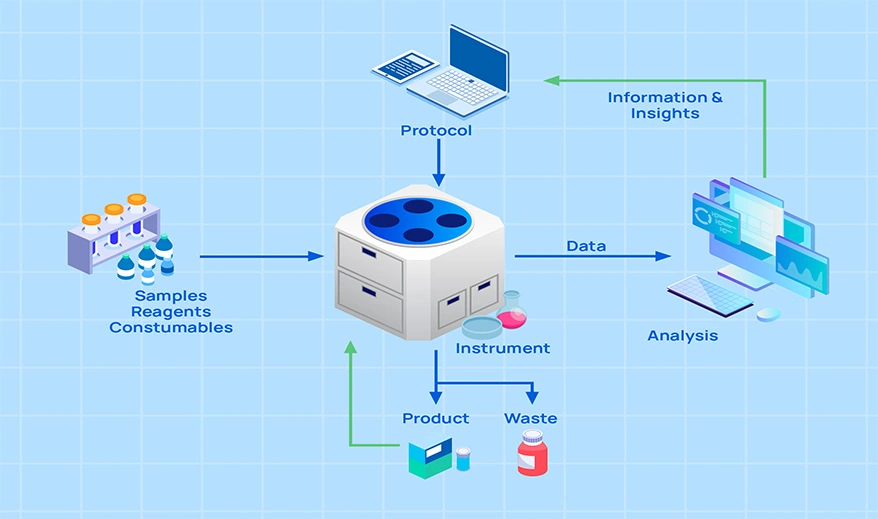
Future pharma-manufacturing lines capture process and product parameters in real-time, disrupting traditional quality control. Distributed QC facilities enable real-time testing on the line, while labs perform specialty testing off-site. Adopting PAT and RTRT requires collaboration with R&D to develop optimal strategies.
Email: info@shentekbio.com
Telephone:
US Mobile: +1-908-822-3199
US Office: +1-800-960-3004 ext 720
China Office: +86-400-878-2189
Address:
USA site: 3350 Scott Blvd Bldg 6, Santa Clara, CA 95054
China site: No.1366 Hongfeng Road, Huzhou, Zhejiang, China, 313000
